"Transparency is becoming less of a value add and more of a table stake. It has to be everyone's aim." — Kevin Johns, Vizient Senior Director, Supply Assurance
James Evans, director of supply chain at Alabama's Springhill Medical Center, sits behind a booth at the Vizient Connections Summit's Member-Supplier Connect. Though supply is a global challenge across the healthcare continuum, he sees plenty of reasons for optimism. Evans has experienced firsthand the resiliency that accompanies increased transparency between providers and suppliers — and there's no reason to assume, he says, that the tide will return to opaque relationships.
"With the issues in supply chain, suppliers are starting to share information and help us get out in front of any problems so we can effectively prepare," he said. "A lot of the prime vendors we deal with are very good at giving us a heads up about what's coming."

To achieve that goal, Vizient has worked to increase transparency among members and suppliers through initiatives such as Vizient Domestic Sourcing, in which members can see two distinctions within the catalog: 1) if products meet Vizient’s “Assembled in USA” standard, which states that the final place of manufacturing is within the U.S. and 2) if they comply with the Federal Trade Commission’s “Made in USA” standard, meaning that all or virtually all of the product has been made in the United States.
In June, Vizient announced a strategic partnership with Supply Risk Solutions to provide a database platform for the collection of healthcare supply pedigree data that aids in supply chain disruption monitoring and risk prevention for members. And last fall, on the heels of bringing 100 million additional units of essential medications to the supply chain through Novaplus Enhanced Supply, Vizient announced the formation of the End Drug Shortages Alliance, an independent organization of stakeholders at all points in the supply chain, to further address disruptions in accessing medications.
"Transparency is becoming less of a value add and more of a table stake," said Kevin Johns, Vizient senior director of supply assurance. "It has to be everyone's aim."
But what exactly does transparency look like across the healthcare continuum? Several providers, suppliers and Vizient experts shared their experiences and best practices at Summit panels, breaking down the new frontier of supply chain considerations.
1) SHIFTING SITES OF CARE
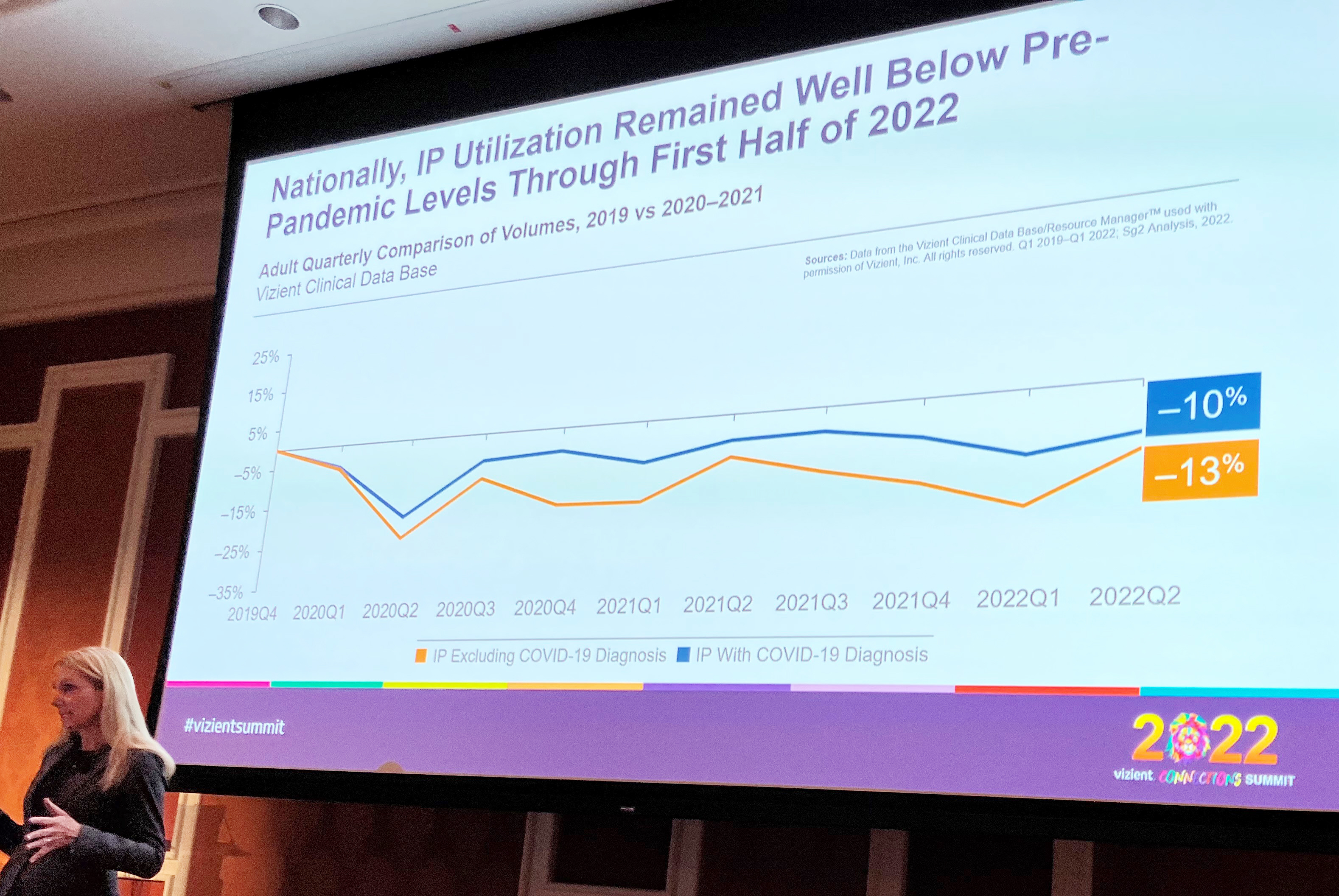
“One of the ways that providers are looking to refocus growth efforts is through diversification and that’s really rebalancing where their historical revenue sources have come from. They’re thinking about providing financial stability and durability moving forward,” said Lisa McGuire, Vizient associate principal, intelligence. “One way to tackle that is thinking about distribution of services. How do providers double down on what they’re really good at?”
Think about service distribution across the various points of care within a five-hospital system, she said. Maybe an orthopedic program at every site isn’t necessary — consolidation at one site of care might be the more strategic approach. As those types of shifts occur, providers must work more closely with suppliers.
“If a health system now only has orthopedics at one site of care as opposed to five, that impacts how they work with Vizient and communicate with suppliers to distribute those supplies in a different way,” McGuire said.
2) LONG-TERM STRATEGY
In the joint member/supplier education session, “Patient Care Utilization Trends and the Impact on Spend Projections,” health systems and their supplier partners agreed on one issue in particular: Transparency can be nerve-wracking, but it’s absolutely critical in today’s constrained supply chain.

That's especially important when it comes to capital, said Dennis Regan, senior director of commercial operations at Stryker.
"Our base disposable and implant business is tied to procedural volume but with capital purchases, there's not a direct procedural volume tie-in," he said. "So, it's more about spend, capital forecasting and lifecycle products. When customers are thinking about updating those, that's the kind of information that would really help inform us on the capital side."
3) TRUST BUILDING
Trust between providers and suppliers was tested during COVID, said Kristine Komives, senior director of supply assurance and procurement at University of Michigan Health, who spoke as part of a pharmacy panel centered on the future of transparency and solving healthcare's toughest challenges. The only way to get back on track is to rebuild that foundation of trust through transparency.
"The remembrance that we're here to help patients has got to lead to better solutions within the supply chain," Komives said. "It leads to the kind of partnership conversations we can have at these conferences, it leads to engagement with Vizient and supply assurance, it leads to open and honest conversations with everyone in that chain. The best conversations we've had is where we've taken our hats off and said, `I'm not the University of Michigan, I'm not my distributor, I'm not my GPO. That's not my role to play.' It is really focusing on that last hundred yards so that the clinicians have what they need to take care of whoever walks through that door. We're motivated by data and transparency every day. We need better information out of the upstream, and we owe suppliers better information about what we're doing in-house."
4) RISK MITIGATION
Over the past three years, Vizient has worked to help its membership and the manufacturing community better understand what the expectations and stakes will be moving forward for better transparency in contracting terms and conditions.
"One thing that is now part of our general template is the concept of a manufacturer letting us know — so that we can in turn let our members know — when they fall below a certain amount of finished goods," said Chad Mitchell, Vizient AVP, contract and program services, who spoke about the supply chain economic outlook and preparing for the unexpected. "This is something that we historically have done as a general practice with our distributors but haven't always necessarily put that same onus on our manufacturers."
Mitchell added that sharing information isn't intended to cause a stockpile situation if a manufacturer falls below that established amount of finished goods.
"It's about sharing data so that we can empower our members to make decisions about when they need to seek alternative product," he said. "The same goes for pedigree. We are asking more and more in our contracts for source of origin and where products are manufactured and kept. And again, that is intended to empower the customer so that they can take alternative measures if needed."
Those who have sought to build increased transparency over the past few years are seeing the payoff when it comes to assessing risk.
"When COVID hit, no one knew where anything was made," Komives said in the pharmacy panel. "When Russia invaded Ukraine this year, I went to our distributor partner, I went to Vizient, and I went to some of our key suppliers and within a day I knew if we were at risk. That's profound movement forward. We forget how good we're getting at this because there's still so far to go. But we have absolutely learned some things as an industry. While we still have geographic concentration in manufacturing that we're trying to address, we're in a much better position than we were in 2019."
