by Gretchen Brummel, PharmD, BCPS, Pharmacy Executive Director, Vizient
Kyle Hoelting, PharmD, BCPS, Senior Clinical Manager of Drug Information, Vizient
Health systems have been inundated with shortages, recently including lorazepam injections, contrast media agents, parenteral nutrition valve sets, electrolytes, bulk fluids, and local anesthetics, among others. Shortages also cross over to other supportive departments such as supply chain and materials management with items such as vials, tubing, syringes and needles.
Drug shortages continue to impact patient care, safety, cost and workforce. While new reported annual shortages peaked in 2011 and have been steadily decreasing since that time, total shortages are on the rise, and the severity of these shortages can be persistently disruptive. Drug shortages can also increase the number and severity of medication errors.
A survey completed by ISMP demonstrated that the majority of respondents felt drug shortages had compromised patient care, and a 2019 literature review found shortages resulted in negative patient clinical, economic and humanistic outcomes. Vizient also completed an impact analysis in 2019 and found U.S. hospitals were spending a combined estimate of $360 million in annual labor costs due to drug shortages.
When a drug shortage occurs, the equilibrium between supply and demand is disrupted. At a local level, actions to correct this imbalance may include consolidating inventory to fewer locations and implementing utilization criteria to specific patient populations. However, such strategies are usually inadequate for prolonged shortages.
Recent significant efforts have been made at a national level to build additional inventory of essential medications and products to create a buffer that could withstand a more enduring disruption of supply. The Food and Drug Administration and other organizations also are advancing efforts to increase awareness and adherence to quality manufacturing strategies. However, as described in the recent National Academics of Science, Engineering, and Medicine (NASEM) report on medical product supply chain resiliency, true success at preventing drug shortages will necessitate a comprehensive, multifaceted strategy to prevent disruption. Rather than relying solely on strategies aimed at product inventory, increasing stewardship, or the quality of medication use, can be another component of a resilient supply chain.
Stewardship by another name
The term stewardship has become increasingly utilized across pharmaceutical care. Simply put, stewardship is when additional attention and monitoring is devoted to a certain category or group of medications to advance better patient care outcomes. Sub-optimal medication use associated with antimicrobial agents – which led to higher rates of resistance, greater medication related adverse effects and additional costs – prompted regulatory and accreditation requirements for antimicrobial stewardship. A historical culture including misuse elevated awareness about appropriate and judicious use of controlled substances in pain management yielding the advent of opioid stewardship. Given their expense, specialty pharmaceuticals are prescribed with thorough utilization management protocols, promoting the stewardship of high-cost medications.
However, for many of the medications that have previously been subject to repeated shortages and used by health systems, such as generic or frequently injectable products, close scrutiny may not occur consistently given the lower cost typically associated with these medications. In this area, applying stewardship practices to shortage management has the potential to positively impact the current state.
Demand reduction as a strategy to resolve a shortage and minimize impact has been described by the National Academies of Sciences, Engineering and Medicine in the past and has worked well locally in mitigating the clinical impact of shortages. A more expansive application to decrease demand at the regional and national levels, coupled with deliberate purchasing behaviors, could yield even greater impact and more rapid resolution of shortages when they occur.
So, what is the one thing you need to know about drug shortage stewardship? Its definition.
Drug shortage stewardship (DSS) is a coordinated program to implement local inventory and mitigation practices to collectively reduce the national demand for the affected drug and limit drug shortage impact. DSS also promotes evaluating use of essential medications, even those for which acquisition cost is not a significant concern, as well as improves medication use and broad access.
At a local level, traditional drug shortage management includes:
- Formation or enhancement of an active multidisciplinary drug shortages committee which may include a pharmacy buyer, inventory manager, compounding manager, pharmacy IT professional, medication safety pharmacist, drug information pharmacist, clinical pharmacists, physician representatives, supply professionals and other experts
- Acute implementation of mitigation strategies such as IV-to-PO conversion or restricting to specific patient populations upon learning of a shortage to decrease the impact
- Development and maintenance of an internal organizational drug shortage list, regardless of the current impact on the organization to improve communication and tracking
- Leveraging regional networks to facilitate improved medication access and collaboration
- Avoidance of gray market purchases and vetting all secondary distributors for questionable practices
Beyond these components, DSS also includes the following to contribute to national solutions:
- Implementation of mitigation strategies even when shortages are not actively impacting the organization
- Continuous review of essential medication prescribing patterns, including low-cost generics, to ensure optimal use regardless of the presence or absence of a supply situation
- Communication mechanisms outside of the organization to convey stewardship actions
- Avoidance of anticipatory purchasing beyond routine or regulatory needs
While most organizations operate under some or all of the tenets of DSS, there is an opportunity to further apply these principles across the broader healthcare system and optimize certain practices that currently unintentionally exacerbate the extent and duration of the supply disruption.
Historically, a heavy focus has been placed on ensuring adequate supply. Activity around demand control consistently occurs at individual organizations through specific mitigation strategies and prudent use guidelines, with less work focused on decreasing regional and national demand; this is the major emphasis of DSS.
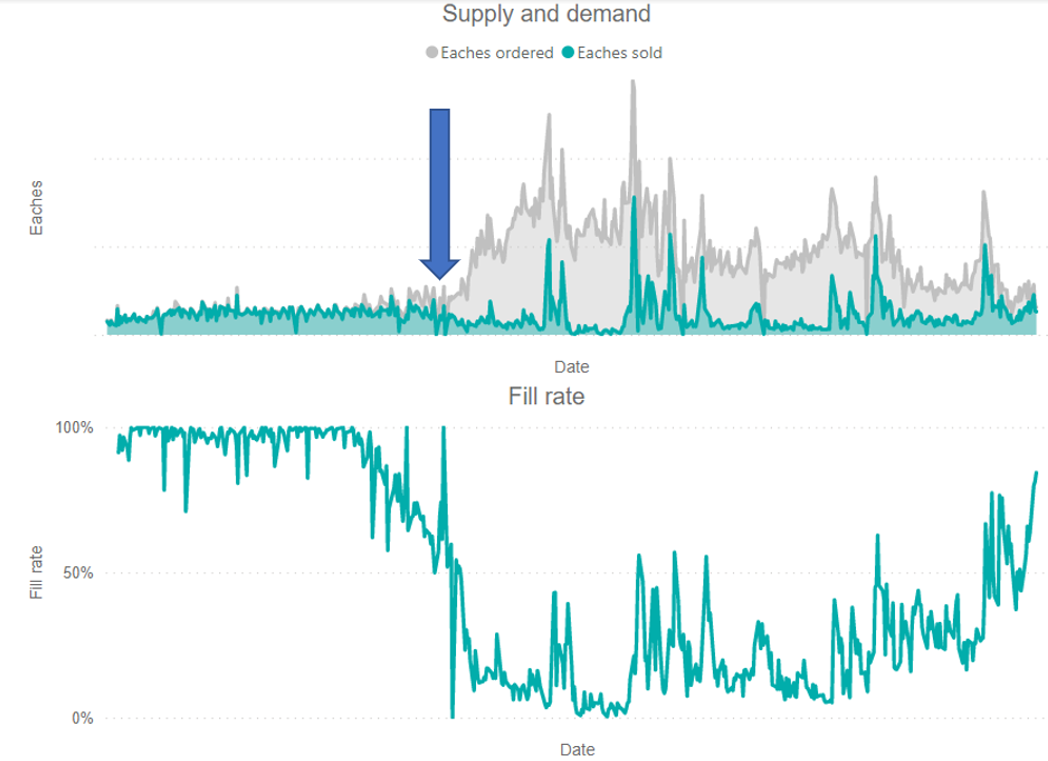
When a critical mass of organizations practice DSS principles, a rapid and potentially significant contraction in demand can occur when a shortage is realized. This demand contraction can “flatten the curve” or “lessen the spike” by heading off anticipatory purchases and limiting utilization on a regional or national basis, allowing the marketplace to recover more rapidly. The blue arrow below in the graph demonstrates where DSS implementation could influence demand spike.
Figure 1. Example of a drug shortage leading to extraordinary increase in demand nationally
DSS will continue to be developed and refined. We introduce it here to facilitate a conversation around DSS concepts and to propose a demand-side national approach as another tool when faced with the ever-present challenge of drug shortages.
Readers are encouraged to share their thoughts, feedback and development suggestions via email at: pharmacyquestions@vizientinc.com.
About the authors
Gretchen Brummel, PharmD, BCPS is a pharmacy executive director at Vizient and provides support to the Center for Pharmacy Practice Excellence team bringing more than 25 years of experience in health care. Gretchen’s areas of expertise include clinical pharmacy services, pediatric pharmacotherapy, medication quality and safety, disaster preparedness and response, medication cost avoidance strategies, drug shortage and formulary management, medication use policy, rural pharmacy practice, clinical research and pharmacy informatics with a focus on pediatrics.
Kyle Hoelting, PharmD, BCPS is a Senior Clinical Manager, Drug Information for Vizient Pharmacy Solutions. In this role he helps to develop evidence-based medicine deliverables, clinical newsletters, and drug shortage mitigation strategies for the pharmacy membership. Kyle earned his Doctor of Pharmacy degree from the University of Nebraska Medical Center. In addition, he completed both PGY1 Pharmacy Practice and PGY2 Drug Information pharmacy residencies at the University of Kansas Health System. Prior to joining Vizient he was the Clinical Pharmacy Specialist, Drug Information and Drug Policy at Virginia Commonwealth University Health. In his previous role, he was responsible for formulary management, drug policy development, evaluation and management of drug shortages, precepting of pharmacy students and residents, as well as facilitating the drug information call center.