- Pharmacy
When Specialty Drugs Are No Longer in the Minority
One of the areas of greatest concern for pharmacy practice, in terms of both cost and impact on patient care, is the increasing development of pharmaceuticals that could be classified as “specialty.” While there is no universally accepted defin...
- Pharmacy
Another Drug Shortage. Another Price Hike?
While attending the National Nuclear Security Administration 2016 Mo-99 conference in St. Louis last week, I learned of a report from the National Academies of Sciences, Engineering, and Medicine (NAS) citing the 50% likelihood of a shortage of molybdenum-...
- Pharmacy
Navigating the Nuances of the Pharmacy Landscape
In my experience on the contracting side of pharmacy, I have yet to see an industry landscape quite as volatile and intriguing as the one we currently find ourselves navigating. As such, Vizient goes to great lengths to predict what’s coming down the...
- Pharmacy
The FAST Generics Act Can Speed Up Pharmaceutical Competition
Given the challenge of achieving agreement on any issue in Washington, D.C., especially on topics involving health care, we should pause and celebrate situations where bipartisan behavior may be taking hold.
- Pharmacy
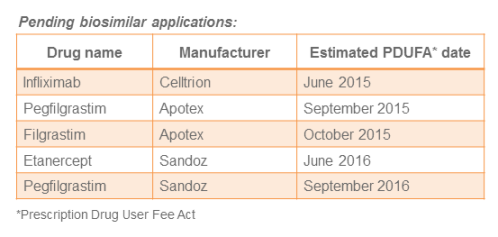
And Then There Were Two: Biosimilars for Infliximab
The FDA recently achieved two significant firsts in one approval. On April 21, the FDA licensed infliximab-abda (Renflexis; Samsung) for marketing. While this product represents the fifth biosimilar approved in the United States, it is the first one licens...
- Pharmacy
The Unintended Consequences of the Orphan Drug Act
If you look up the word “orphan” in the dictionary it is commonly used to convey loss and abandonment. However, when orphan is used in the pharmaceutical industry, it conveys dollar signs. Big dollar signs.
The world’s 10 most expensive ...
- Pharmacy
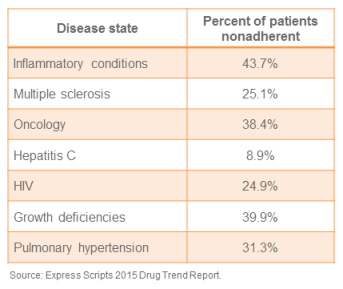
Specialty Pharmacy: A Revenue Center in Disguise
Here’s a scenario: Department leaders across the health system are meeting to look for untapped sources of incremental revenue. They hope to find a traditional cost center that could become a strategic asset. An added bonus would be if it also enabled them...
- Pharmacy
Recognition is Nice, but Accuracy is Most Important with Biosimilars
It was a pleasant surprise to see not one, but two recent commentaries on the subject of biosimilars in prestigious publications such as the New England Journal of Medicine (NEJM) and the Journal of Clinical Oncology (JCO). Given the novelty and complexity...
- Pharmacy
Making the Case for Biosimilars
I recently had the pleasure and privilege to represent Vizient and present at a public hearing by the U.S. Food and Drug Administration (FDA), focused on removing barriers affecting the uptake of biosimilars, which offer a cost savings for some of the most...
- Pharmacy
Understanding the Real Impact of Recent Drug Pricing Announcements
In July, several major pharmaceutical companies responded to President Trump’s call to action to lower drug prices by announcing they would freeze price increases through the end of 2018. While much was made in the media about the effectiveness of th...
- Pharmacy
How to Manage the Ongoing Fragility of the Nuclear Supply Chain
Last week, we received news of an industry-wide shortage on vital radiopharmaceuticals, Tc-99m and Mo-99, due to international reactors being shut down for scheduled and unscheduled maintenance. While we believe the shortage will be temporary – leadi...
- Pharmacy
Facing the Challenges of the Plasma Market
Plasma-derived therapeutics are part of a complex dynamic of global supply chain economics and clinical contributing factors, all of which can affect the ability to obtain the product needed for patient care. Immunoglobulin (Ig), the main driver in current...
- Pharmacy
Maintaining the Integrity of High Consequence Spaces
There are areas in health care facilities that require specific environmental controls and operational procedures to ensure safety, such as patient isolation rooms, surgical suites and compounding areas. Environmental or procedural breaches in these &ldquo...
- Pharmacy
Understanding the Potential Impacts of USP <825> for Radiopharmaceutical Compounding
In recent years, there has been a significant amount of regulatory focus on compounding pharmacies. Specifically, regulatory agencies want to establish guidance for quality and sterility of products with the goal of providing the highest standard of finish...
- Pharmacy
USP Regulatory Updates Delayed on Appeal
We’ve recently shared communications about updates to existing United States Pharmacopeia Convention (USP) Chapters surrounding pharmaceutical compounding regulations as well as a new regulation for handling hazardous drugs in health care facilities....
- Pharmacy
COVID-19 Response Requires Innovation from Hospital Labs: 4 Actions to Take Now
At the beginning of the COVID-19 pandemic, medical laboratories immediately experienced a decline in testing volume. And, while the test volumes are inching upward as labs find their footing in the new pandemic environment, the rate of recovery is slower t...
- Pharmacy
Our National Lab Experiment: Testing for the SARS-CoV-2 Coronavirus
As a nurse with decades of experience, I have been perplexed by the ongoing reporting challenges related to testing for COVID-19. There are a multitude of vendors providing the various tests that determine if a patient is infected with the active virus and...
- Pharmacy
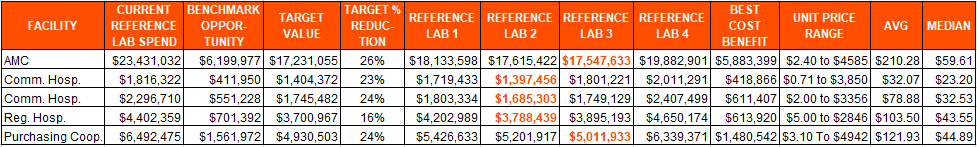
Getting the Best Pricing for Reference Laboratory Services
The COVID pandemic has produced many new challenges for hospital-based laboratories. One area that laboratory leaders may find immediate cost reduction opportunities is through a review of utilization and pricing for reference laboratory services.
- Pharmacy
Managing COVID-19 Testing Demands in your Health System: Key Considerations for CFOs Before Investing in New Technology
The drum beat for managing the COVID-19 pandemic from public health leaders continues to be test, test, test. Before investing in new technology, there are several considerations that chief financial officers should consider.
- Pharmacy
Use of Monoclonal Antibody Therapy Continues to Advance
One of the more recent advancements in pharmaceuticals has been transformative to the management of disease is the discovery and utilization of “monoclonal antibodies” (mAb). Its impact cannot be overstated. Learn more about how mAbs are also playing criti...
- Pharmacy
It’s Time for Commonsense Drug Pricing Solutions
A cheer went up among Vizient and our members last year when HHS announced the termination of the FDA’s Unapproved Drugs Initiative to curb rising drug costs. Our analysis found that ending the program could protect the U.S. health care system from an addi...